Biography: I am a pharmacy student at University College London with a strong interest in…

Cancer Viro-Immunotherapy: Repurposing the Past to Shape the Future
Authors: Rosie Mundy and Aimee Lucignoli
Viruses have a long history of causing illness and disease, but a recent BSGCT blog post highlighted how they can also be used for good (https://www.bsgct.org/post/making-gene-and-cell-therapy-possible-viruses). By engineering, mutating and even a bit of tinkering we can manipulate the infection mechanism of a virus to serve a new purpose, for example, to treat patients by delivering gene therapies accurately where they are needed. There is a long list of ongoing clinical trials using engineered viruses, with many focusing on engineered viruses as cancer vaccines to stimulate anti-tumour immunity(1). However, cancer immunotherapies can be expensive, in addition to taking a long time to develop, so researchers have needed to get inventive(2).
We are not seeing the first cases of trying to treat cancer with immunotherapy. In the 19th century, Dr. William Coley would inject cancer patients with a combination of heat-killed bacteria, named Coley’s toxins. His toxins stimulated the immune system and often were successful, with some other medical professionals at the time also adopting this method(3). Ultimately there were difficulties in reproducing Coley’s toxin recipes exactly and administering them to patients. Radio and chemotherapies also began to emerge at this time and so the first cancer immunotherapy was pushed to the side-lines. Immunotherapy has now become much more popular, and we are gradually revisiting Coley’s approach as he was correct – some cancers are sensitive to non-specific immune activation within the tumour, rather than relying on initial recognition of the tumour itself.
With this in mind, a different strategy is emerging in pre-clinical testing. The repurposing of some licensed viral vaccines, which would usually be injected intramuscularly to stimulate a systemic immune response, could see their benefits as cancer therapies using an intratumoral route of administration(4). It is hypothesised that the immune response would be focused on the tumours, attracting the attention of the immune system to an immune-sparse, immunologically “cold” environment. This has been tested with an inactivated influenza vaccine as demonstrated below(5).
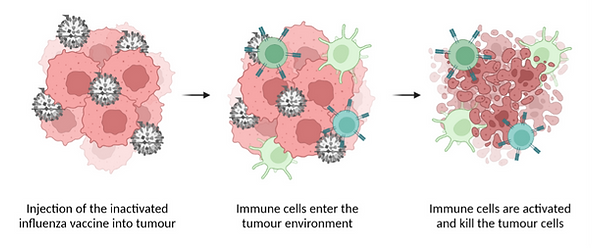
Intratumoral injection of influenza vaccine as a cancer immunotherapy. The seasonal flu vaccine contains inactivated influenza virus (grey). Tumour cells are pink. Once injected into the tumour, the virus particles interact with dendritic cells (light green) which results in increased antigen presentation and more CD8+ T-cells (darker green) in the tumour microenvironment, including some specifically targeting tumour cells. The strong immune presence and activation reduces tumour growth. It also enhances tumour sensitivity to checkpoint blockade immunotherapy in the pre-clinical model. (Figure created with BioRender.com)
Making use of licensed vaccines would widen the somewhat limited accessibility to immunotherapy for many cancer patients(2). The costs of bringing a brand new therapeutic through the drug development pathway are acknowledged to be astronomical and this traditional pathway can be longwinded. Re-testing a licensed vaccine for alternative use would cut down on this time, building on an already obtained safety profile, and therefore greatly reducing the cost(6). It is still important to note that repurposed vaccines would need regulatory approval with associated clinical evaluation before becoming routinely used in the clinic as a cancer therapy. This does come with a price tag, however, the overall cost is still much less than developing a completely new therapy. Finally, there are many licensed vaccines available across the globe with the potential to be repurposed, helping ease the burden of accessing immunotherapy for cancer patients internationally.
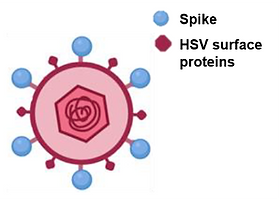
Nevertheless, one of the main limitations found in in vivo pre-clinical testing of repurposed vaccines is the requirement for intratumoral delivery. This can be an issue for hard-to-reach cancers, like pancreatic, or for patients with metastases. Some research groups have taken the approach of utilising a commonly used, well-characterised virus and re-targeting it to produce a new virus which will selectively infect cancer cells(7). An alternative approach to circumvent the delivery problem was recently demonstrated in a pre-print article by Yaping Sun et al. The authors produced an oncolytic Herpes-Simplex virus and engineered it to wield the spike protein from SARS-CoV-2 on its surface(8). The virus’ replication had been restricted to tumour cells only, so can be administered intravenously and attract the immune system to the tumour site in addition to working as a vaccine against SARS-CoV-28. It is worth noting, however, that when combining Coley’s ideas with an engineered virus, we may lose the benefits that come with repurposing existing vaccines, such shortened timelines and reduced costs.
The majority of research into repurposing vaccines as cancer immunotherapies has so far been performed in the lab, but there has also been some success for repurposed vaccines in clinical trials. An influenza vaccine is currently in a phase I clinical trial as a combination therapy for abdominal cancer patients undergoing surgery(5,9) and, although it is not a viral vaccine, the BCG (Bacillus Calmette-Guérin) bacterial vaccine should not be overlooked when discussing repurposed vaccines as it has high reported success rates for urological tumours(10). We will have to wait patiently for the results of additional clinical trials looking at other licensed vaccines(9), but for now, it is clear that these vaccines have much more potential than their current use.
The repurposing of viral vaccines marks a revitalising period for cancer immunotherapy, an area frequently expensive and difficult to access for patients. While it is unlikely to be a magic cure-all for every case, the repurposing of pre-existing vaccines has the potential to open up immunotherapy for cancer patients globally. Perhaps the most interesting point is that this approach is a nod to the birth of cancer immunotherapy, revisiting early ideas with the improved knowledge and understanding that many researchers (including many in this society!) have worked hard to compile.
References:
1. Liu, J., Fu, M., Wang, M., Wan, D., Wie, Y. and Wie, X. 2022. Cancer vaccines as promising immunotherapeutics: platforms and current progress. Journal of hematology and oncology 15(1), pp. 28-28. doi: 10.1186/s13045-022-01247-x
2. Pantziarka, P., Verbaanderd, C., Huys, I., Boucher, G. and Meheus, L. 2021. Repurposing drugs in oncology: From candidate selection to clinical adoption. Seminars in cancer biology 68, pp. 186-191. doi: 10.1016/j.semcancer.2020.01.008
3. Tontonoz, M. 2015. What Ever Happened to Coley’s Toxins? Cancer Research Institute. https://www.cancerresearch.org/en-us/blog/april-2015/what-ever-happened-to-coleys-toxins.
4. Russel, S.J., Peng, K.-W. and Bell, J.C. 2012. Oncolytic virotherapy. Nature Biotechnology 30(7), pp. 658-670.
5. Newman, J. H. et al. 2020. Intratumoral injection of the seasonal flu shot converts immunologically cold tumours to hot and serves as an immunotherapy for cancer. Proceedings of the National Academy of Sciences 117(2), pp. 1119-1128.
6. Plotkin, S., Robinson, J. M., Cunningham, G., Iqbal, R. and Larsen S. 2017. The complexity and cost of vaccine manufacturing – An overview. Vaccine 35(33), pp. 4064-4071. doi: 10.1016/j.vaccine.2017.06.003
7. Davies, J. A. et al. 2021. Efficient intravenous tumor targeting using the αvβ6 integrin-selective precision virotherapy ad5null-a20. Viruses 13(5), p. 864. doi: 10.3390/v13050864
8. Sun, Y. et al. 2021. Dual roles of a novel oncolytic viral vector-based SARS-CoV-2 vaccine: preventing COVID-19 and treating tumor progression. bioRxiv, p. 2021.2006.2007.447286.
9. Vandebourne, L., Panziarka, P., Van Nuffel, A. M. T. and Bouche, G. 2021 Repurposing Infectious Diseases Vaccines Against Cancer. Frontiers in oncology 11, pp. 688755-688755. doi: 10.3389/fonc.2021.688755
10. Lamm, D. L. and Morales, A. 2021. A BCG success story: From prevention of tuberculosis to optimal bladder cancer treatment. Vaccine 39(50), pp. 7308-7318.



