The BSGCT Conference celebrated the path of research that led to today’s ground-breaking treatments. As…

Researcher Spotlight – Dagmara Szmaglińska – Winner of the BSGCT Scientific Writing Competition
From Lab to Clinic: The Science Behind Safer Stem Cell Therapies
Imagine a future where damaged organs are repaired, blindness is reversed, and Parkinson’s disease treated. Sounds like science fiction? It’s not! Current advancements in regenerative medicine are getting us closer and closer to achieving this in real life. One of the most promising forms of regenerative medicine is stem cell therapy, which is based on stem cells that can be grown infinitely in the laboratory. These powerful cells can become any cell type in the human body and grow indefinitely in the lab (Figure 1), providing an enormous supply of building blocks that can be used as a basis for therapies and opening up new, exciting opportunities in modern medicine. In the future, it may be possible to treat some diseases not just by easing their symptoms using pills, but by replacing faulty cells with healthy ones made in the lab.
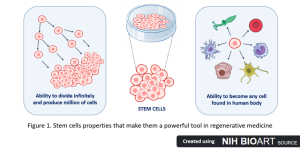
With great power comes great responsibility
As scientists have got closer to bringing stem cell-based treatments to patients in clinical trials, a key safety red flag has been raised: the emergence of genetic variants. Those variants are lab-grown cells that have undergone small changes in their DNA. Because the order of the DNA enables cells to make proteins with important tasks, any damage to DNA can have negative consequences. Think about a recipe: even small, unwanted changes that go unnoticed could disrupt it. For example, just one letter mistake from ‘salt’ to ‘malt’ will completely ruin your meal. The same goes for producing cell therapy products from variant stem cells.
So what can we do to ensure the treatments we’re using to heal people don’t end up causing harm? As scientists are getting to know more about the origin, impact, detection, and prevention of variants, their discoveries are raising new questions. How do variants appear? Why do the DNA changes matter? What are their consequences? How do we find harmful variants? How do we stop or reduce variant stem cells?
When stem cells go wrong
Imagine you’re growing a beautiful flower bed. You’ve carefully planted flowers and maintained them, but unexpectedly, weeds start growing as well. Left uncontrolled, these weeds can crowd out your flowers and take over the garden. Something similar happens when growing stem cells in the lab – an essential step in making stem cell therapies (Figure 2). Unlike the human body, the lab environment, while controlled, is not the cells’ natural habitat. Over time, the stress of being artificially grown in a dish and repeatedly handled to propagate and multiply the cell numbers can lead to changes in the cells’ DNA.
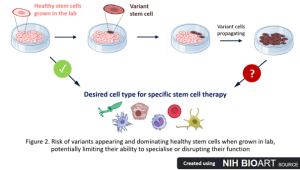
These DNA changes aren’t random (1). They appear, again and again, in different labs and stem cells from various sources. Such changes suggest that certain variants help the cells survive and grow better in the lab (2, 3), just like weeds that thrive in disturbed soil (Figure 2). These variant cells might grow faster, live longer, or ignore signals that tell them to die, while under some stressful environmental conditions, like higher DNA damage. Unfortunately, the same traits and DNA changes can also be found in some cancer cells (4, 5). If these cancer-like variants aren’t detected and removed, they could take over healthy cells and jeopardise the safety and effectiveness of the therapy. Although variants are usually found in only 25% of lab-cultured stem cells (1, 4, 6), we don’t yet know the therapeutic consequences they might have.
The responsibility of monitoring the genetic health of stem cells
While this area is actively being explored, our limited understanding calls for close monitoring of lab-grown cells and continued research. Firstly, it’s important to establish which genetic changes we need to watch out for. Experts in the field have raised a call for action (7), which includes a substantial variant library to be created from all stem cell researchers worldwide. We can establish guidelines and predict risky variants, but only through collaborative actions. Once we know which variants are dangerous, we need appropriate tools to spot them while cells are grown in the lab. This is of utmost importance before we can even begin to think about using these stem cells for treatments.
There are several methods to check the whole genetic material of cells using sequencing, like checking each letter of a word in a recipe before it’s published. However, analysing all 3 billion building blocks called nucleotides is expensive and needs specialist expertise. Assuming an average of 500 words per page and 5 letters per word, 3 billion nucleotides would translate into about 600,000 pages, and even one letter change could make a cell into a variant! This shows how crucial the right tools are to catch these changes early on.
As stem cells grow and divide fast, they have to replicate these nucleotides in around 20 hours every time they divide. Sounds like a lot to handle, right? That’s why scientists are also looking into optimising the environment and habitat of these cells. Some progress has already been made in this area, and the percentage of variant cells growing in the lab has decreased. For example, it has been achieved by growing cells in lower oxygen conditions (8, 9), which resemble a natural habitat, or by tweaking the composition of the cell food solution that the cells are grown in (10). Understanding the mechanisms of how exactly these variants appear and what these environmental pressures are can therefore help us find optimal conditions for growing stem cells in the lab.
Stem cell therapies offer a powerful new approach to treating the root cause of diseases that were once considered untreatable. By closely monitoring the genetic health of stem cells in the lab and improving the conditions in which they grow, scientists are working towards safer, more effective stem cell therapies. While there are still challenges and knowledge gaps to overcome, each discovery brings us closer to life-saving stem cell therapies for patients who currently lack treatment options.
References:
- Stavish D, et al. Feeder-free culture of human pluripotent stem cells drives MDM4-mediated gain of chromosome 1q. Stem Cell Reports. 2024;19(8):1217-1232. doi:10.1016/j.stemcr.2024.06.003
- Price CJ, et al. Genetically variant human pluripotent stem cells selectively eliminate wild-type counterparts through YAP-mediated cell competition. Dev Cell. 2021;56(17):2455-2470.e10. doi:10.1016/j.devcel.2021.07.019
- Draper JS, et al. Recurrent gain of chromosomes 17q and 12 in cultured human embryonic stem cells. Nat Biotechnol. 2004;22(1):53-54. doi:10.1038/nbt922
- Lezmi E, Jung J, Benvenisty N. High prevalence of acquired cancer-related mutations in 146 human pluripotent stem cell lines and their differentiated derivatives. Nat Biotechnol. 2024;42(11):1667-1671. doi:10.1038/s41587-023-02090-2
- Delbany D, et al. De Novo Cancer Mutations Frequently Associate with Recurrent Chromosomal Abnormalities during Long-Term Human Pluripotent Stem Cell Culture. Cells. 2024;13(16):1395. Published 2024 Aug 21. doi:10.3390/cells13161395
- Keller A, et al. Uncovering low-level mosaicism in human embryonic stem cells using high throughput single cell shallow sequencing. Sci Rep. 2019;9(1):14844. Published 2019 Oct 16. doi:10.1038/s41598-019-51314-6
- Benvenisty N, et al. A call to action for deciphering genetic variants in human pluripotent stem cells for cell therapy. Cell Stem Cell. 2025;32(4):508-512. doi:10.1016/j.stem.2025.03.003
- Thompson O, et al. Low rates of mutation in clinical grade human pluripotent stem cells under different culture conditions. Nat Commun. 2020;11(1):1528. Published 2020 Mar 23. doi:10.1038/s41467-020-15271-3
- Kuijk E, et al. The mutational impact of culturing human pluripotent and adult stem cells [published correction appears in Nat Commun. 2020 Aug 4;11(1):3932. doi: 10.1038/s41467-020-17797-y.]. Nat Commun. 2020;11(1):2493. Published 2020 May 19. doi:10.1038/s41467-020-16323-4
- Halliwell JA, et al. Nucleosides Rescue Replication-Mediated Genome Instability of Human Pluripotent Stem Cells. Stem Cell Reports. 2020;14(6):1009-1017. doi:10.1016/j.stemcr.2020.04.004


